Danielle Freeman ’17 Explores Relationship between Sunlight and Oil Spills
By Jane Godiner ’23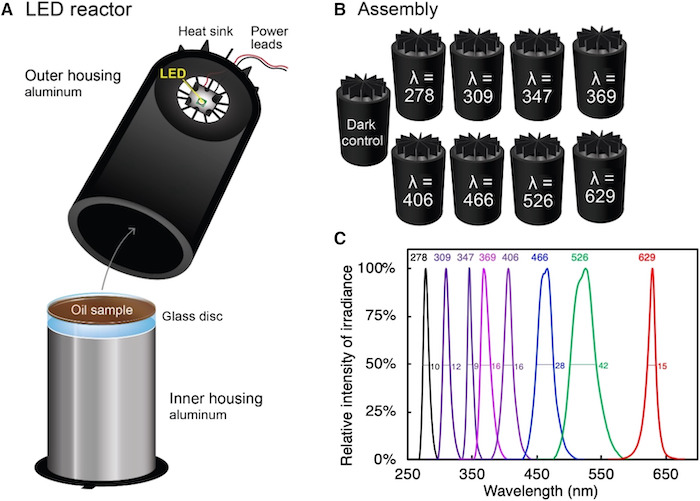
A diagram of the apparatus used to analyze the oil samples, as displayed in Freeman's article, "Sunlight-driven dissolution is a major fate of oil at sea."
"For this project, we wanted to investigate the chemical transformation of oil by sunlight, which can create new compounds that dissolve in seawater rather than remain in the floating oil slicks at the sea surface," Freeman said in a recent interview. "We call this process photo-dissolution."
Her research contributes new information to the growing body of knowledge about the phenomenon and impacts of photo-dissolution.
"Scientists have known for fifty years that photo-dissolution affects oil, but no one knew how much," Freeman said. "That’s why we set out to quantify how fast oil photo-dissolution happens, and to use our results to calculate the impact that the process likely had during the 2010 Deepwater Horizon oil spill in the Gulf of Mexico, and could have in future spills."
While Freeman was extremely motivated to do this work, she and her co-author, Collin Ward—as well as other staff, students, and faculty at Woods Hole Oceanographic Institution (WHOI)—were challenged by the circumstances in which they had to conduct the research.
"This research was conducted during 2020 and 2021, during a time when we had limited lab access at WHOI," Freeman said. "It took a lot of coordination, long hours alone in the lab, video calls and outdoor meetings in all weather to get this project done! Looking back, it’s amazing that things came together as well as they did."
Ultimately, the results of Freeman's research revealed not only how fast photo-dissolution happens, but its significance in industrial disasters.
"Our experimental results showed that photo-dissolution happens fast—fast enough that it could have removed almost 10 percent of the surface oil during the Deepwater Horizon spill," Freeman said. "We also found that sunlight-driven reactions can be important in other spill scenarios besides Deepwater Horizon. For example, we found that these reactions could be important during the summer in the Arctic Ocean, where the risk of future oil spills is increasing due to higher ship traffic in an increasingly ice-free summer sea."
Freeman looks forward to doing more research on the phenomenon of photo-dissolution, emphasizing that there are still many more exciting aspects of the process to uncover.
"We don’t know yet whether the net effect of oil photo-dissolution is good or bad after an oil spill—it could have some positive and some negative effects at the same time," Freeman said. "This is often the kind of result we get in science, and I know it can be frustrating!"
"What our results do offer is a way to quantify a process that hasn’t been quantified before, so that the community of scientists and first responders working on oil spills are better prepared to account for this process, understand its effects, and use resources effectively after a spill."
In the wake of her recent publication, Freeman reflects fondly on and believes strongly in the value of her Bowdoin education—as well as her time spent as a laboratory instructor in the Bowdoin chemistry department—in helping her access her career.
"It’s a testament to the value of a liberal arts curriculum and the quality of Bowdoin teaching that these classes led me to a career path I never would have otherwise imagined," Freeman said. "What I love now about my work is that it allows me to think creatively about the world around us while at the same time answering research questions that have real-world applications to protect ecosystems and the humans who rely on them."