Plant growth, morphogenesis and development involves cellular adhesion, a process dependent on the composition and structure of the extracellular matrix (ECM) or cell wall. Pectin in the cell wall is thought to play an essential role in adhesion, and its modification and cleavage are suggested to be highly regulated so as to change adhesive properties. To increase our understanding of plant cell adhesion a population of EMS mutagenized Arabidopsis were screened for hypocotyl adhesion defects using the pectin binding dye Ruthenium Red that penetrates defective but not WT hypocotyl cell walls. We isolated numerous mutant lines and my lab is currently characterizing these.
The ELMO Family
 Genomic sequencing was used to identify a mutant allele of ELMO1 which encodes a 20 kDa Golgi membrane protein that has no predicted enzymatic domains. Mutants resemble the Sesame Street character ELMO when stained with Ruthenium Red. ELMO1 colocalizes with several Golgi markers and elmo1-/-plants can be rescued by an ELMO1-GFP fusion. elmo1-/-exhibits reduced mannose content relative to WT but no other cell wall changes and can be rescued to WT
Genomic sequencing was used to identify a mutant allele of ELMO1 which encodes a 20 kDa Golgi membrane protein that has no predicted enzymatic domains. Mutants resemble the Sesame Street character ELMO when stained with Ruthenium Red. ELMO1 colocalizes with several Golgi markers and elmo1-/-plants can be rescued by an ELMO1-GFP fusion. elmo1-/-exhibits reduced mannose content relative to WT but no other cell wall changes and can be rescued to WT 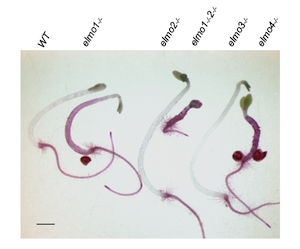 phenotype by mutants in ESMERALDA1 that also suppresses other adhesion mutants. elmo1 describes a previously unidentified role for the ELMO1 protein in plant cell adhesion. ELMO1 is a member of a family of 5 ELMO-like proteins all predicted to be in the Golgi.
phenotype by mutants in ESMERALDA1 that also suppresses other adhesion mutants. elmo1 describes a previously unidentified role for the ELMO1 protein in plant cell adhesion. ELMO1 is a member of a family of 5 ELMO-like proteins all predicted to be in the Golgi.

We are investigating if these small non enzymatic proteins serve a scaffolds for pectin biosynthesis enzymes, and are required for correct pectin deposition and hence adhesion.
The Cytoskeleton and Pectin Based Adhesion
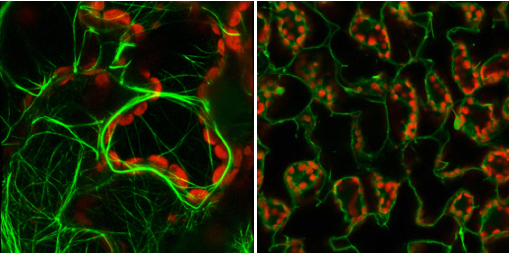
The pectin-rich plant cell wall defines cell structure and facilitates cell adhesion. A screen of mutant Arabidopsis hypocotyls identified a new allele of QUASIMODO2 (QUA2), a gene required for pectin accumulation and cell adhesion. A suppressor of qua2 was isolated and describes a null allele of SABRE (SAB), which encodes a previously described plasma membrane protein required for longitudinal cellular expansion that organizes the tubulin cytoskeleton. sab mutants have increased pectin content, increased levels of Pectin 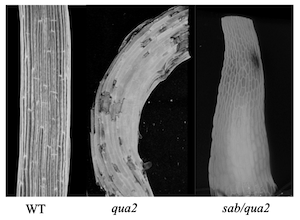 Methyl Esterases, Extensins, Arabinogalactan Proteins, and reduced cell surface area relative to qua2 and WT, contributing to a restoration of cell adhesion. Thus, the tubulin cytoskeleton influences the accumulation of cell wall pectin, proteins, and cell adhesion.
Methyl Esterases, Extensins, Arabinogalactan Proteins, and reduced cell surface area relative to qua2 and WT, contributing to a restoration of cell adhesion. Thus, the tubulin cytoskeleton influences the accumulation of cell wall pectin, proteins, and cell adhesion.
The Plant Cell Wall: Wall Associated Kinases
The plant cell wall is secreted and assembled by cells such that it can provide structure and shape, and thereby help to determine the form of a plant organ. Control of the synthesis and directional enlargement of the wall is therefore crucial for plant development, but the wall also serves as a first defense against common plant stresses such as pathogens and physical wounding. My lab discovered the vascular plant Cell Wall Associated Kinases, WAKs, and over the last twenty years has helped to establish that they are pectin receptors required for both normal cell elongation and for an induced stress response.
The Plant Cell Wall
Cell walls differ greatly between species and cell types, but the primary wall or region that is first laid down seems to have a similar basic underlying architecture. A rosette of plasma membrane cellulose synthases extrudes cellulose polymers into the extra cellular space, resulting in intertwined cellulose fibers of varying complexity. Cellulose synthase is associated with the cytoskeleton which helps in its directional synthesis. The cell wall contains a number of other sugar-based polymers, such as hemicellulose and pectin both of which are synthesized in the golgi and secreted via vesicles. Regulation of the direction and time in which the wall is synthesized and expanded can therefore dictate cell size and shape and organ characteristics, since the cell wall restricts the outward forces of cellular turgor.
Pectins in plants form a jelly like matrix in which cellulose, hemicellulose and a variety of other carbohydrate and proteins are embedded and collectively are termed the plant cell wall. Pectins are chains of α-(1-4)-linked D-galacturonic acid, forming polymers of homo galacturonic acid (HG) of 100-200 residues. Pectins are present in variable lengths in the cell wall, but are fragmented during wounding or pathogen presentation, and these fragments are termed oligogalacturonides or OGs. Some have suggested OGs play a fundamental role during developmental processes, and there is abundant literature that suggests OGs stimulate a specific cytoplasmic response, including the activation MAPKs and numerous defense related genes, and an accumulation of ROS. Until recently the existence or identity of a pectin or OG specific receptor was unknown.
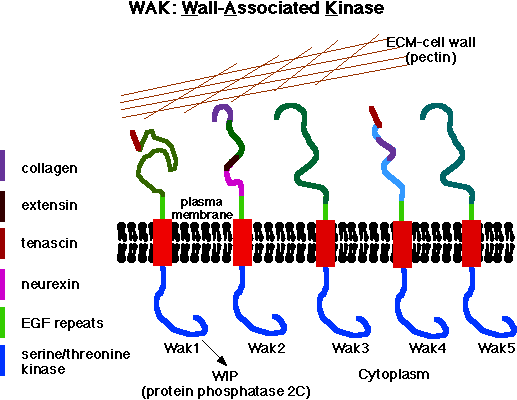
WAKs Are Pectin Receptors
Over the past few years, we and one other lab have established that the Arabidopsis Wall Associated Kinases or WAKs are pectin receptors. The WAKs of Arabidopsis bind to pectin in the extracellular space, traverse the plasma membrane, and have a functional serine threonine kinase domain in the cytoplasm.
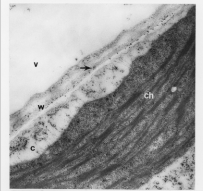
Electron Micrograph with gold labeled WAK on cell surface and cell wall
WAKs are bound to pectin in native cell walls and their activity is required for normal cell expansion, yet OGs also bind to WAKs and mediate a response to pathogens and wounding. Our current model is that the type and concentration of pectin present in the wall leads to a WAK-dependent activation of different signaling pathways. Unchallenged but expanding walls would preferentially activate, via WAKs, a cell expansion path that includes Mitogen Activated Protein Kinase 3 (MPK3). When OGs are generated by a wall disturbance, the WAKs may alter their signaling path to help effect the stress response by now also activating MPK6 and a new downstream response. That the in vitro binding assays reveal a higher binding affinity of OGs than longer polymers for WAK suggests a mechanism by which WAKs can switch from binding the native cell wall pectin to OGs, thus distinguishing types of pectin. Differential activation by various pectins might be achieved by the specific pectin affinity of an individual receptor, or perhaps combinations of WAKs with as yet unidentified partners.
TGFß like receptors of the Chloroplast, Mitochondrion, and Plasma Membrane
In the 1990s my lab discovered a family of Arabidopsis receptor-like protein kinases that we initially thought were only active in the thylakoid membrane of the chloroplast. These receptors were identified through a biochemical screen for a protein that phosphorylated the light harvesting antennae (LHC) of photosystem II, and the family was termed TAK for Thylakoid Associated Kinase. We established that indeed TAK1 and TAK2 were required for LHC phosphorylation, and this is important as phosphorylation induces the migration of the LHC between PSI and PSII so as to balance the energy input. Most fascinating it that these receptors have strong structural similarity to the developmentally important TGFb family of metazoans, including alternatively spliced messages that encode inhibitory receptor domains, and a common dimeric, co-receptor activation method. We then realized that the TAK family contained 7 members, some of which were instead localized to the mitochondrion, and to the plasma membrane. Single mutations in TAKs had little phonotypic effect, yet triple mutants appear to create female gametophyte lethality; the pollen germinate and meet the egg yet are unable to correctly fertilize. Our goal is more fully characterize this TAK family, and to establish the respective role of each member that is differentially localized in the cell.

Kohorn on stage at right winning the 1st Annual Ben and Jerry's Ice Cream Eating Contest, Burlington VT, 1978