Plant Biologist Bruce Kohorn and Students Discover (Curly, Red) Clues to Cell Wall Adhesion
By Rebecca Goldfine
Kohorn, an accomplished scientist with many published articles, has made discoveries that have influenced the world of plant science. But in the case of his new research, he credits his laboratory team with their contributions to the achievement. "The real story here is the students," he said.
In the past two years, despite the disruptions and restrictions of the pandemic, five students have helped push Kohorn's lab into new scientific terrain.
Frances Zorensky ’20, Jacob Dexter-Meldrum ’20, and Bridgid Greed ’20, worked with Kohorn last year, up until they had to abruptly stop their experiments in March when the campus closed. This year, biology major Wesley Hudson ’21 and biochemistry major Andy Bolender ’21 picked up where Zorensky and Dexter-Meldrum left off.
The work precipitated by Zorensky and Dexter-Meldrum is featured in two forthcoming articles: one in Development, and the other in Plants. Greed's work was just published in PLOS One.

New Players in Cell Adhesion
With genetics, microscopy, and biochemistry, Kohorn's lab studies the cell walls of vascular plants, including how cells adhere to one another. Plant cells are knitted together into an "extracellular matrix," which is mostly made of cellulose and pectin deposited on the outside of cell walls. These walls perform three essential tasks: they maintain the structure of the cells and of the plant, abet plant growth and development, and guard against pathogens.
Kohorn was led into this area of research while pursuing his decades-long work on the WAK receptors for pectin, which are essential for plant development and resistance to pathogens. Greed's work on this topic showed that these receptors are important for the production of reactive oxygen species that help fight pathogens, but also interact with molecules important for adhesion.
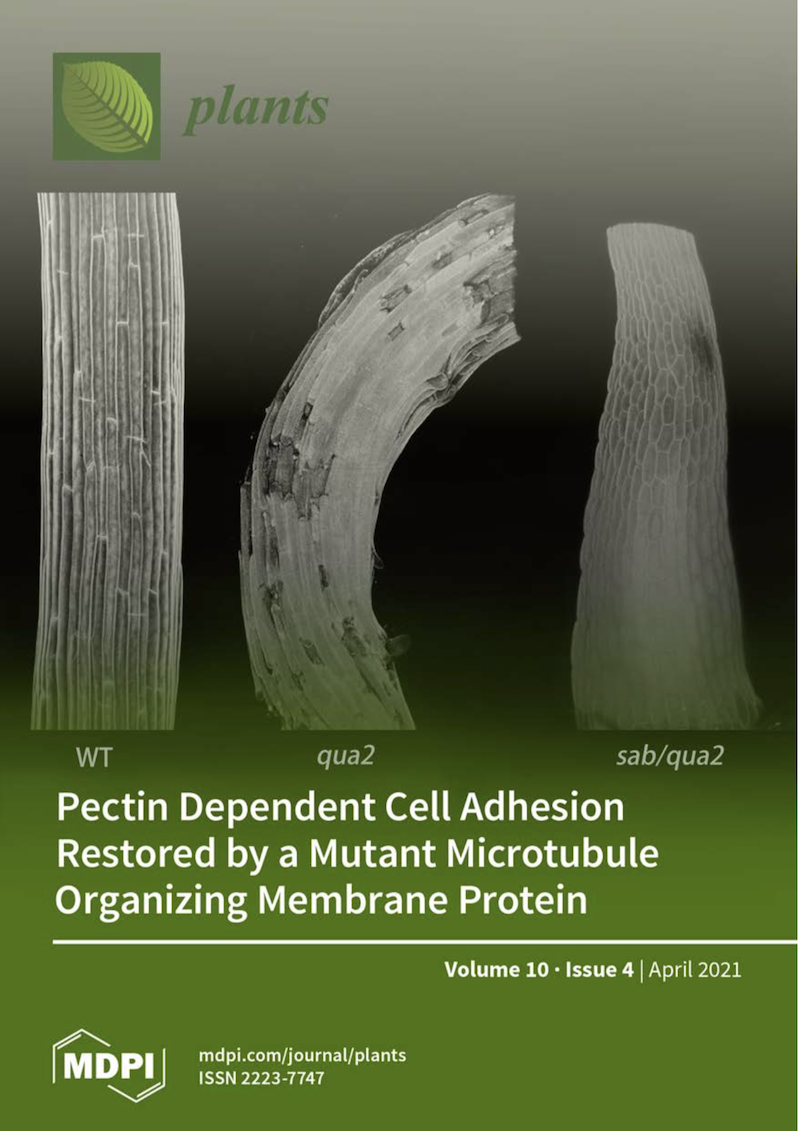
In the recent Plants article, Kohorn and his co-authors describe finding a new mutation in a gene responsible for synthesizing pectin, which acts like the primary glue between cells. But they also found a secondary mutation that fixes this error by altering the cystokeleton of the cell. Kohorn credits Dexter-Meldrum for "astutely noticing" this suppressor mutation.

A stained red seedling, indicating an Elmo mutant.
And in the Development article, the authors explain how a previously uncharacterized gene plays a vital role in cellular adhesion.
"One of the most characteristic aspects of a plant is how its cells sticks together and how it maintains a stiff wall structure, so we're getting at the nitty-gritty of plants in a way," Hudson said.
These discoveries are exciting because they move scientists closer to fathoming the complex and dynamic mechanism of cellular adhesion in plants. "There have to be processes that are very controlled to stick and unstick cells," Bolender said. "These processes change over a plant's life, and a cell's life. And they're different in different parts of the cell. For example, in the root tip you have cells multiplying, but in the stem, the cells grow and elongate."
To hone in on the specific genes responsible for cellular adhesion, Kohorn's team first looked for physical deformities in their model plant Arabidopsis. "If you find a plant that has a mutation somewhere that stops that cell adhesion, you can identify the gene that is mutant and see what that gene codes for," he said. "That tells you something about how adhesion is being regulated and how it works."
Following on work started on Kohorn's sabbatical in Versaille, Zorensky and Dexter-Meldrum began screening thousands of Arabidopsis seedlings for mutant plants. Under a microsope, they looked for signs of cell walls fraying or curling. If the result was too subtle to observe, they used a red dye that can penetrate cell walls and expose imperfections. If a tiny seedling grew up with red stains, it indicated problems.
Zorensky was the first in the lab to identify a gene that disrupted cell wall adhesion. "The one that I ended up finding was in a gene that had not previously been characterized in any sort of way," she said. "Its role was unknown." She proposed calling it Elmo (see sidebar).
This year, Hudson and Bolender continued the investigation into Elmo, with both producing "great honors theses," Kohorn said. They ended up identifying a handful of related homologs to Elmo I, and have characterized an additional four so far, Elmo 2 through 5. These are proteins that look similar in structure to Elmo 1.
Hudson found that while two of the Elmo mutations on their own have a weak or no impact on cellular adhesion, doubling them up produces a crippling effect on the plant. "This indicates they're redundant," Korhon said, "and are doing similar things in the cell."

In the tradition of geneticists giving quirky names to new mutants, Zorensky decided that the mutatations resembled the cute, giggly Sesame Street character, Elmo.
"Being born in 1998, I looked at the mutant—which stains red and has these cells curling off the side because they’re not adhering properly—and I thought of Elmo, who is red and curly," she explained.
She added: "It's such a wild feeling that as students, we found this gene and named it, and made an impact in science that feels high level."
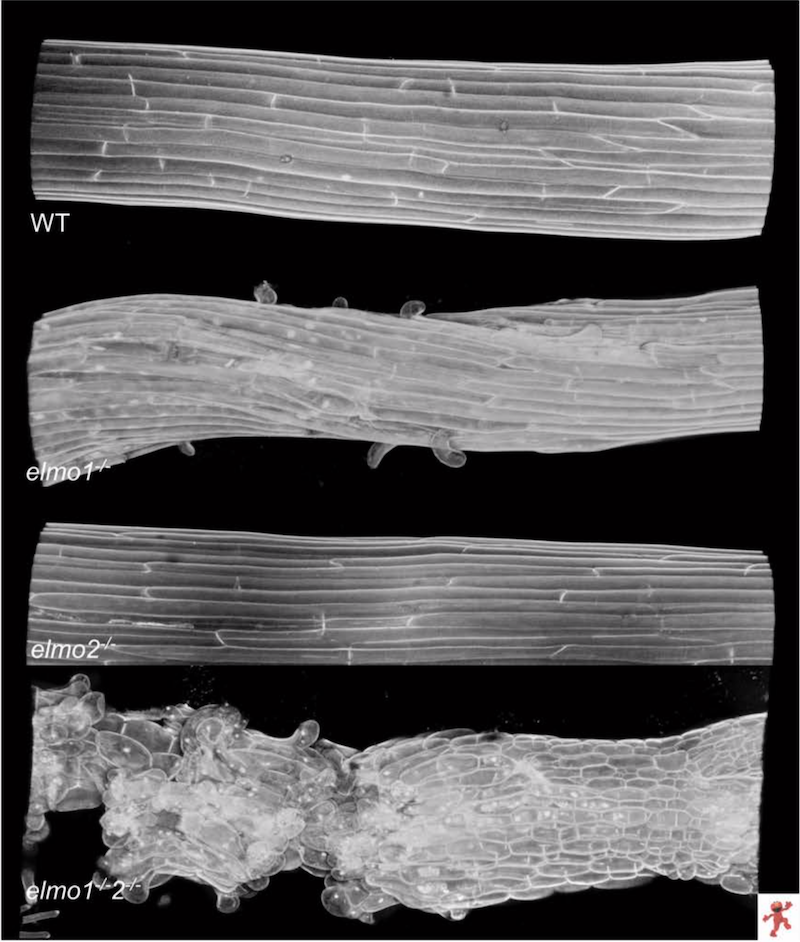
There are several reasons why a gene might have copies, other than possibly being a way to ensure that a mistake or mutation is not lethal. Another is that an organism might want to turn the genes on and off at different times during development, Bolender explained. Or, a gene might get duplicated through mutation, but without any negative consequences, so that duplication gets passed on to subsequent generations.
Part of Bolender's honors project research was locating two of the Elmo homologs—Elmo 2 and 3—in the cell. Through a long, complicated process, he was able to pinpoint their home in the Golgi apparatus, which is where pectin is synthesized. Elmo I is also in the Golgi.
"Knowing where proteins are in a cell has a big impact on what functions they can perform," Bolender said, and what their relationship is to one another. His finding supports the idea that Elmo genes are redundant, or partially redundant.
The team also believes at this point that the Elmo genes don't actually synthesize molecules but rather scaffold together other molecules so they can make pectin more efficiently.
Bolender also investigated another mutation the lab identified that was affecting cellular adhesion, and revealed that cellulose was likely involved. Cellulose is what gives cell walls their rigidity. Up to now, it was considered to play no role in cell wall adhesion. Yet, "there is something about the architecture of the cell wall that cellulose contributes to that is necessary for cellular adhesion," Bolender said.
The Joys of Basic Science
Kohorn pursues his research because he's "interested in understanding how things work," he said. Whether that information later becomes integral to agriculture or biofuel development is not what motivates him.
His students agree that doing research into the fundamental laws of nature is exciting, even if its future impact or applicability is uncertain at these early stages. "I think the appeal of basic science is that eventually something will pop—it could be a new technique that comes from a basic discovery. Part of this is science for science's sake, and that adds value to the greater scientific community," Hudson said.
After graduation, Hudson will work as a research technician at the Ragon Institute in Cambridge, Massachusetts, focusing on HIV immunotherapy research. He, Bolender, and Zorensky all say they would like to go to medical school one day.
Though they're shifting their attention to humans and away from plants, the three say they're grateful for their time in Kohorn's plant biology lab.
"I have had a great time," Hudson said. "It's deepened my understanding of how to take a research question and follow it all the way through to the end."
Bolender also acknowledged the importance of botany to human biology. "A lot of our modern understanding of how biology works comes from plant researchers," he said. "And lots of plant research has been very important for increasing food production and addressing hunger," he added.
Zorensky said the experience in Kohorn's lab went far beyond showing her how to do basic research; it shifted the way she thinks about medicine, community, and her possible role in health care. "It has taught me a lot and provided me with a perspective of the world I didn’t expect to gain," she said.
"You’re looking at these cells and how each of them interacts with and supports each other. And the connections they have affects the individual cell as well as the organism—its growth and development. It got me thinking about what that looks like on the human scale, especially around community engagement and health. Our actions impact each other directly, and I want to figure out how to best channel my knowledge, and apply my curiosity for biology and research, at a community level," she said.